 |
Data Integrity ALCOA++ defines a framework to achieve data integrity, especially important for regulated industries. |
Data integrity can be thought of as a component of a larger process ensuring the security, traceability, and quality of an organization's results over their whole lifecycle. The principles outlined in ALCOA, ALCOA+, and ALCOA++ support efforts toward data integrity and include ensuring that data is attributable and traceable, among others.
Based on the ALCOA, ALCOA+, and ALCOA++ framework we have developed a poster to help ensure data integrity in every laboratory.
Enhancing Data Management and Integrity in Laboratories, Universities, and Companies
Laboratories, universities, and companies increasingly focus on improving data management and integrity in their daily operations. A critical component of this effort is the connectivity provided by technologies such as Wi-Fi, Ethernet, and Bluetooth®, which link lab equipment, technicians, and students, enhancing collaboration and efficiency.
Data can be collected either manually or electronically. Regardless of the method used, it is vital that all records remain original. Transferring manually recorded data into a spreadsheet can introduce significant risks if the information lacks proper attribution. Additionally, data validity may be compromised when employees sign for others, records are incomplete, or data security is insufficient.
The ALCOA++ Data Integrity principles are established guidelines from the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and other regulatory bodies. These principles are specifically designed to tackle the challenges of maintaining data integrity, especially in the context of electronic records.
The acronym ALCOA++ defines a comprehensive framework for achieving data integrity, which is crucial for regulated industries.
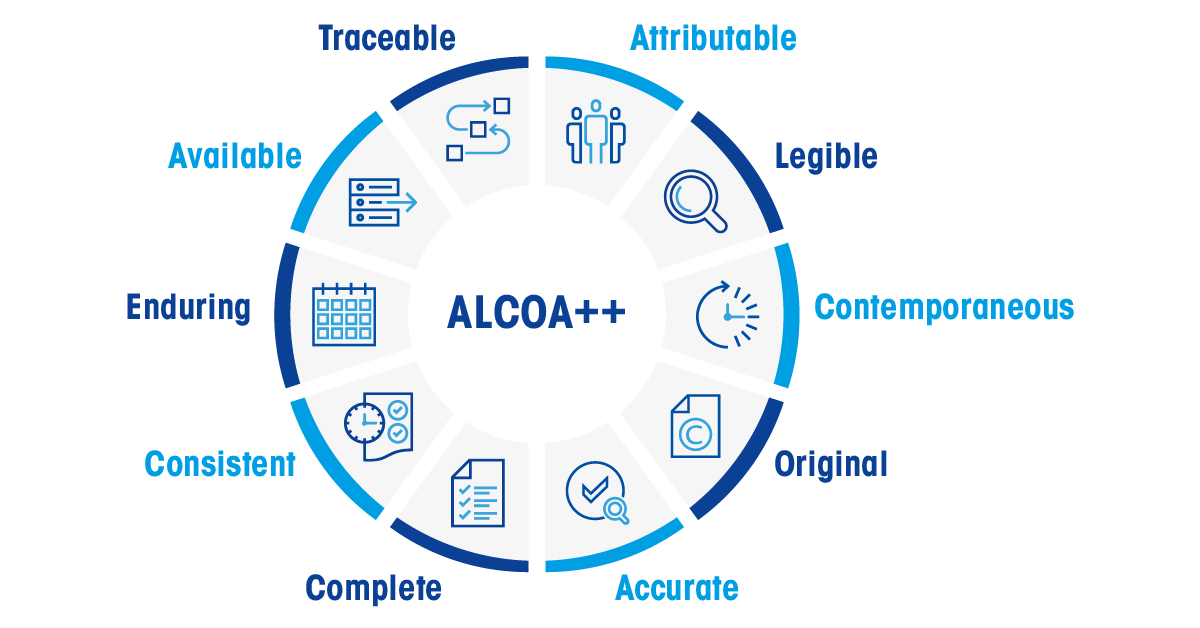
The core principles of ALCOA are as follows:
Attributable: Record the individual or system responsible for each action performed.
Legible: Ensure that data is readable throughout the entire lifecycle of the record.
Contemporaneous: Document data during the activity to reflect true conditions.
Original: Maintain records as original documents or certified true copies.
Accurate: Avoid editing errors without appropriate documentation of amendments.
In addition to these core principles, the following criteria are essential for maintaining data integrity:
Complete: Document all data, including tests, repetitions, or reanalysis, to ensure thorough records.
Consistent: Follow a systematic approach in documenting all analysis components, ensuring a logical sequence of events.
Enduring: Create sustainable records that are systematically documented within a validated system.
- Available: Ensure data can be accessed for review, audits, or inspections throughout its entire lifecycle.
By integrating these principles, organizations can effectively comply with ALCOA+ standards for data integrity. Recently, EMA guidelines on computerized systems have introduced a tenth criterion, further refining the ALCOA framework to ALCOA++:
- Traceability: Ensure that data is traceable throughout the entire process and its lifecycle, including all changes made.
Implementing the ALCOA++ principles is essential for organizations striving to uphold the highest standards of data integrity. This will ensure the reliability and compliance of their operational practices.
Enhancing data management and integrity can significantly improve the operational efficiency and regulatory compliance of laboratories, universities, and companies.