Regelefterlevnad och dataintegritet
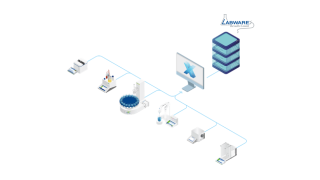
LabX™ stöder regelefterlevnad och dataintegritet genom att kombinera robusta verifieringskedjor med förbättrad datahantering för full spårbarhet, i enlighet med ALCOA++-principerna.
Redo för revision, när som helst!
Vi erbjuder snabb och enkel åtkomst till alla data, med centraliserad användarhantering, elektroniska signaturer och omfattande verifieringskedjor, i linje med bestämmelser som 21 CFR Part 11.