The Elements of Good Pipetting Practice
METTLER TOLEDO Rainin offers a broad range of training and resources to help bench scientists, lab managers, and quality and safety professionals achieve more reliable and repeatable experimental results while lowering their organization’s overall risk.
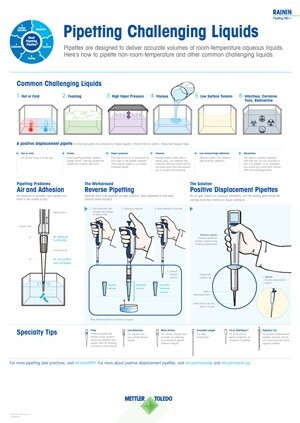
Choose the right pipettes and tips for your workflow by using our risk-based approach to protocol evaluation and instrument selection.
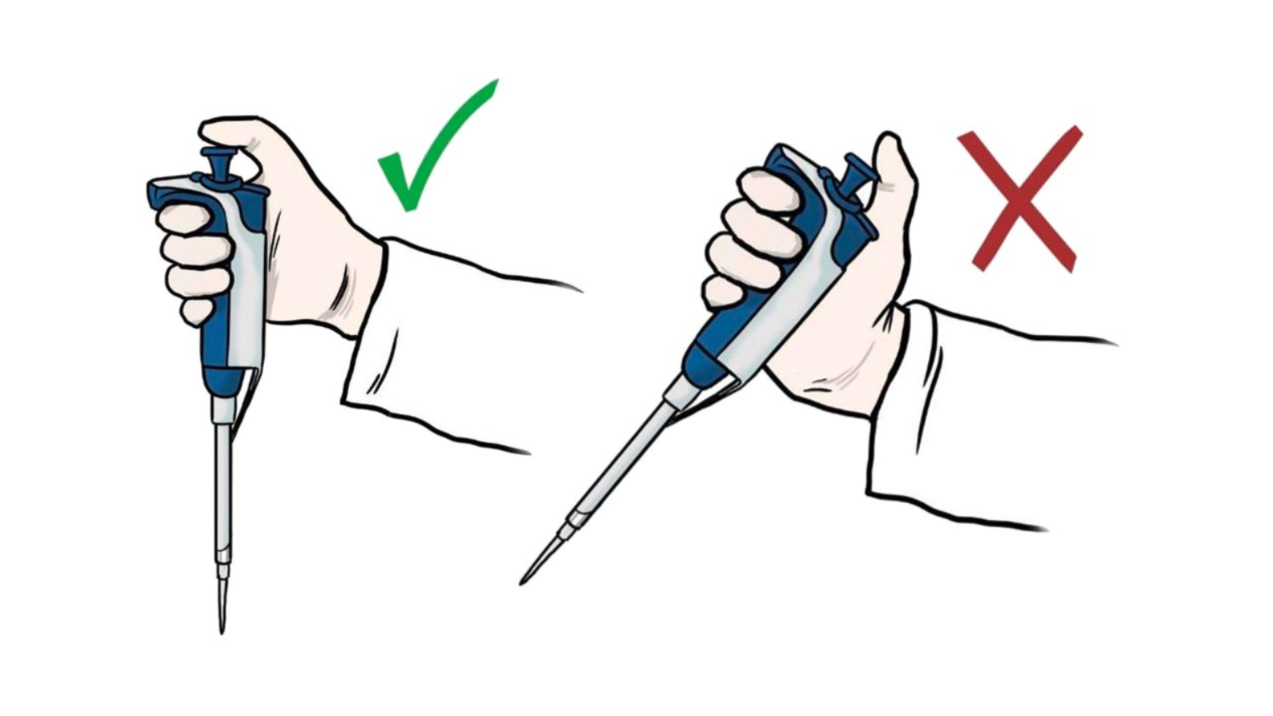
Improve pipetting skills and accuracy by focusing on how techniques influence pipetting performance and experimental reproducibility.
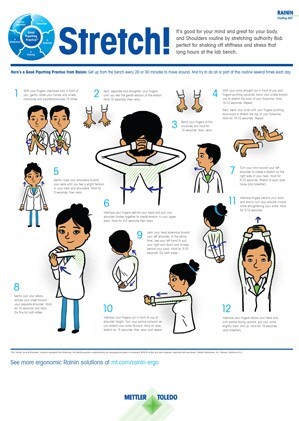
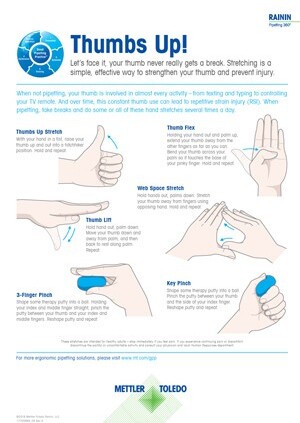
Good bench ergonomics enhance health and well-being while improving experimental reproducibility and reducing the potential for repetitive stress injuries.
Quality assurance needs vary greatly across labs and organizations. GPP can help you find the right balance between quality, security, compliance, and cost.
To help you achieve reliable and accurate results, we consolidated these principles and insights from our pipetting experts into a handbook. This handbook is a comprehensive resource for pipette users at all levels of experience.


















.jpg.crop.0_000000.0_117925.100_000000.99_764151.0_7092198581560284.jpg/_jcr_content/renditions/cq5dam.web.1280.1280.jpeg)


.jpg.crop.0_000000.0_058962.116_666667.116_391509.0_7092198581560284.jpg/_jcr_content/renditions/cq5dam.web.1280.1280.jpeg)

.jpg.crop.0_000000.0_000000.100_000000.100_000000.1_7777777777777777.jpg/_jcr_content/renditions/cq5dam.web.1280.1280.jpeg)