Our focus here is a major pharmaceutical organization that supplies generic medicines and biosimilars worldwide. With expertise spanning prescription drugs, OTC medicines, and antibiotic production, the company has a strong research-and-development track record—especially in the field of oncology support therapies. However, their existing instruments and manual data procedures weren’t keeping pace with evolving compliance demands, particularly around pH and conductivity measurements based on USP <645>.
Challenges
- Outdated Data Capture: Creating workflows and maintaining digital records that align with data integrity requirements was cumbersome on older devices.
- Complex Sample IDs: Managing up to 20–30 samples daily overwhelmed manual entry processes, raising the risk of transcription errors.
- Multi-Step SOPs: Reliance on memory for intricate test sequences exposed the lab to out-of-spec results and potential regulatory flags.
- Inefficient Monitoring: Without centralized oversight, investigating deviations was tedious—any slip in SOP adherence could trigger a lengthy internal probe.
- Paper Journals: Old-fashioned logging introduced further transcription hazards, which didn’t meet modern standards for data reliability.
Solutions: SevenExcellence + LabX
To overcome these issues, the company chose SevenExcellence meters paired with LabX™ software for data management and workflow integration.
Seamless Data Acquisition
With LabX coordinating every step in the background, operators can calibrate the pH meter, record results, and generate compliant reports without juggling separate programs. This alignment with USP <645> ensures measurement uniformity while drastically reducing manual burdens.
Automation Yields Efficiency
Ultrapure water analysis under USP <645> involves multiple stages—each prone to user fatigue if done by hand. LabX solves this by letting labs design customized workflows, turning those multi-step SOPs into guided prompts that minimize error risk.
 |
Figure 1: Optimized conductivity measurement: SevenExcellence meter combined with LabX software streamlines the pro- cess and reduces error margin. |
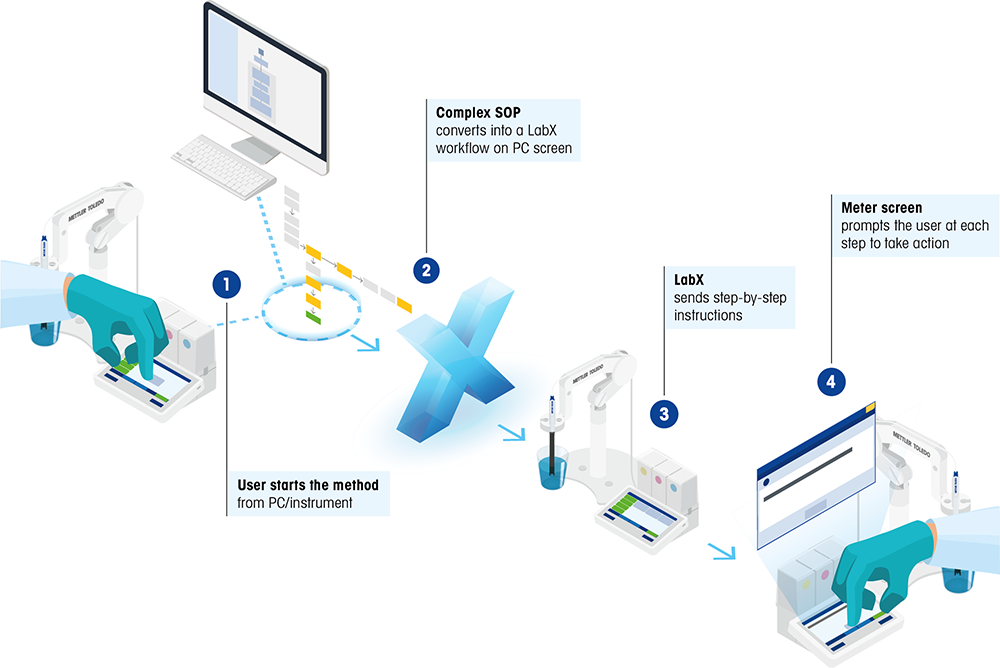 |
Figure 2. Improved efficiency: Converting complex SOPs into simplified and efficient work- flows. |
Reliable, Accurate Results
Every action taken at the instrument terminal or PC is logged automatically, ensuring a clear audit trail. Different user-access levels help guard against out-of-spec results, guaranteeing no unauthorized changes.
Pre-checks in LabX force operators to calibrate sensors within valid ranges—cutting down on questionable data and re-runs.
Noteworthy Outcomes:
- Better Compliance: LabX software with SevenExcellence fosters data integrity—meeting or regulatory standards.
- Single Source of Truth: Converting SOPs into LabX workflows ensures universal guidance at the instrument terminal, removing guesswork.
- Greater Flexibility: Users can operate from the meter or PC, with automatic data storage in the background.
- Reduced Errors: Digitizing the entire process lessens transcription issues, helps standardize procedures, and keeps the lab audit-ready.
Conclusion & Impact
By uniting SevenExcellence meters with LabX software, this global pharma site overcame outdated manual workflows—cutting down on error-prone data entry and ensuring consistent, validated steps for each measurement. Thanks to integrated prompts, robust audit trails, and real-time data capture, their lab is now set up for greater efficiency and unwavering compliance with USP <645> and beyond.
 |
On-Demand Webinar: pH Measurement and Compliance
Ensure your pH measurements stand up to industry regulations and deliver consistent product quality. This concise webinar covers segment-specific guidelines (Pharma, Food & Beverage, Chemicals), essential instrument specs, and expert tips on accurate, repeatable, and reliable pH testing. Join us to learn how compliance pitfalls can be avoided, how best practices boost measurement confidence, and why the right instrumentation is key.
 |
Parul Chhaparia |
Parul Chhaparia is a Content and Marketing Communications Specialist at METTLER TOLEDO, where she crafts impactful campaigns that bridge scientific expertise with engaging storytelling.
With a background in business journalism and extensive experience in creating content that simplifies complex scientific topics, Parul combines analytical precision with creative flair. Beyond her work at METTLER TOLEDO, she enjoys traveling to uncover stories about people, cultures, and places, fueling her passion for discovery and connection.






