When it comes to meeting 21 CFR Part 11 requirements, the importance of data integrity assurance is understandably very high. Yet, a surprising number of labs still assume that instruments running on “firmware-only” can deliver the same level of compliance as a fully computerized system. In reality, these standalone setups carry hidden vulnerabilities—from incomplete metadata capture and manual transfers to risky storage methods that leave labs open to error and regulatory scrutiny.
In this special report, we draw insights from our whitepaper, Electronic Records on Instruments: The Concept of 21 CFR Part 11 Without a PC, which features expert commentary from industry veteran Bob McDowall. You’ll discover why firmware-based record-keeping often falls short, how ALCOA++ guidelines can illuminate hidden compliance gaps, and what you can do to ensure robust data oversight in your lab. Read on for an in-depth preview, then download the whitepaper for the full picture.
 |
Firmware-Only “Compliance”: A Hidden Gamble
A growing number of labs are asking whether instruments without connected software can legitimately meet 21 CFR Part 11 requirements. The whitepaper lays out a cautionary message: while standalone systems might appear cheaper and simpler at first glance, they often fail to capture essential details like user IDs, time stamps, raw data, and complete audit trails.
“Certification of analytical instruments with only firmware has weaknesses and cannot be justified,” Bob McDowall says, underscoring the inherent risk in these so-called “certified” instruments.
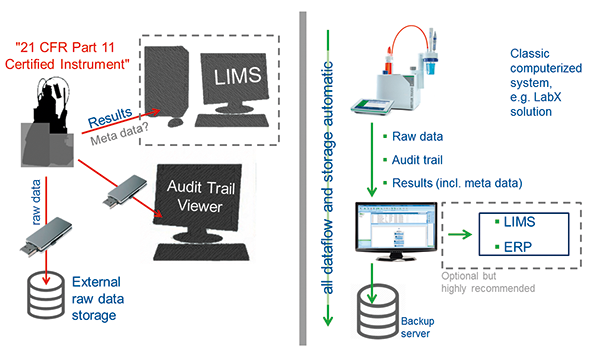 |
Figure 1: Scheme on the left shows a possible data flow for a 21 CFR Part 11 certified instrument. Risky manual data transfers via USB sticks can cause significant data weaknesses and bear the risk of losing data. The scheme on the right shows the data flow with LabX®, which is easier, automatic and secure. |
Key Concerns:
- Data Loss: Limited on-board memory demands frequent manual transfers, often via USB sticks—a process prone to errors, manipulation and deletion.
- Incomplete Metadata: Passing only the final measurement to LIMS or ERP solutions can omit vital context (e.g., calibration data, user credentials). Measuring into compliance is a real concern.
- Uncertain Audit Trails: If raw data and audit logs aren’t readily traceable and verifiable, regulatory authorities can question the authenticity and completeness of the results.
ALCOA++ Under the Microscope
The white paper introduces the foundational GMP concept of ALCOA—Attributable, Legible, Contemporaneous, Original, Accurate—and expands it to ALCOA+, which adds “Complete” as a crucial requirement for data integrity. Labs often underestimate the challenge of satisfying these standards when storing data exclusively on firmware-based instruments—particularly over multiyear lifespans, during firmware updates, or following hardware replacements. Ensuring that all raw measurements, re-analyses, and audit trails remain intact and retrievable is far more difficult when standalone systems handle records with minimal metadata or no centralized archival.
Beyond ALCOA+, industry practices now refer to ALCOA++, which folds in “Consistent,” “Enduring,” and “Available” to address the full spectrum of contemporary compliance needs. While the white paper focuses primarily on ALCOA/ALCOA+ to illustrate how standalone firmware might fall short, ALCOA++ underscores the same vulnerabilities more sharply: if your data isn’t securely archived, fully traceable, and easily accessible over time, it falls short of today’s heightened regulatory expectations. Taken together, these principles stress that truly complete data oversight requires more than local memory or a printed readout—it demands an integrated, software-based approach to meet today’s exacting compliance and security standards.
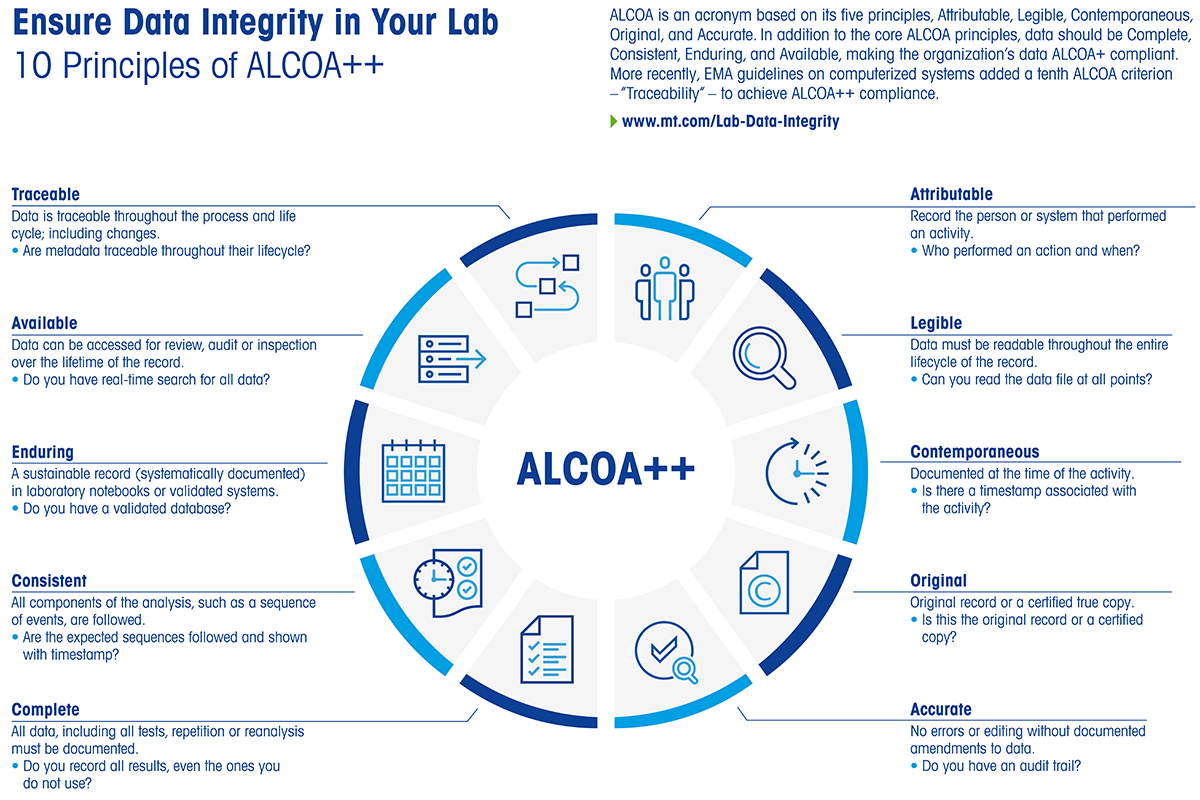 |
Figure 2: The ALCOA++ Principles – download our breakdown of these principles here.
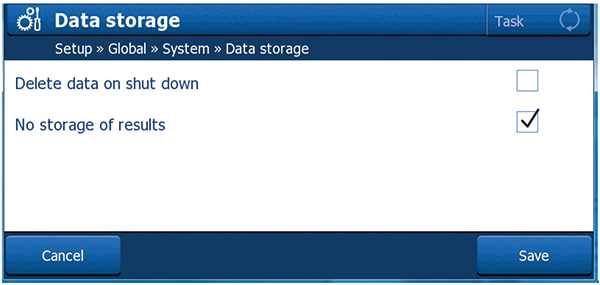 |
Figure 3. Newer analytical instruments from METTLER TOLEDO have the option of turning off the storage of results at the instrument. After generating a result, it gets printed but not stored. Since only one original exists, it can be qualified as a Group B instrument. |
Group B vs. Group C: What USP <1058> Really Says
Another misconception highlighted in the whitepaper is the idea that less validation is required for standalone instruments simply because they’re considered “Group B.” In reality, once electronic records and audit trails enter the mix, USP <1058> (2017 version) classifies such instruments as Group C—requiring full validation akin to a computerized system. The time and costs saved in bypassing a PC can quickly evaporate when labs face unplanned compliance deficits or system reworks.
Some labs prefer an instrument to be qualified as Group B under USP <1058>. For that reason, certain METTLER TOLEDO models include an option to disable local result storage, printing the measurement immediately instead. In this mode, only one “original” exists: the physical printout. However, while this configuration meets Group B criteria, it does not align with the robust data controls of 21 CFR Part 11, which requires secure, traceable electronic records and audit trails—typically achieved via software like LabX®.
The Total Cost of Ownership (TCO) Equation
Although buying a firmware-only instrument appears cost-effective, the whitepaper emphasizes the importance of looking at the Total Cost of Ownership (TCO). Manual data handling, repeated validations, and the expense of investigating potential integrity breaches can swiftly outweigh any upfront savings. By contrast, implementing a robust electronic data management system—such as LabX® software—streamlines workflows, reduces error risk, and automates data capture and storage.
Advantages of a Software-Integrated Setup:
- Automated Data Capture, Synchronization, and Backup
(No more manual USB transfers or piecemeal record-keeping—data is automatically stored in a secure database.) - Streamlined Audit Trails with Centralized Storage
(Real-time audit logs are accessible and tamper-evident, minimizing regulatory hurdles.) - Easier Second-Person Reviews and Regulatory Inspections
Auditors can quickly trace data origins, modifications, and user actions—reducing compliance risks. - Seamless Connectivity to LIMS, ELN, or ERP
By including metadata and timestamps, labs ensure final results remain both verified and thoroughly documented.
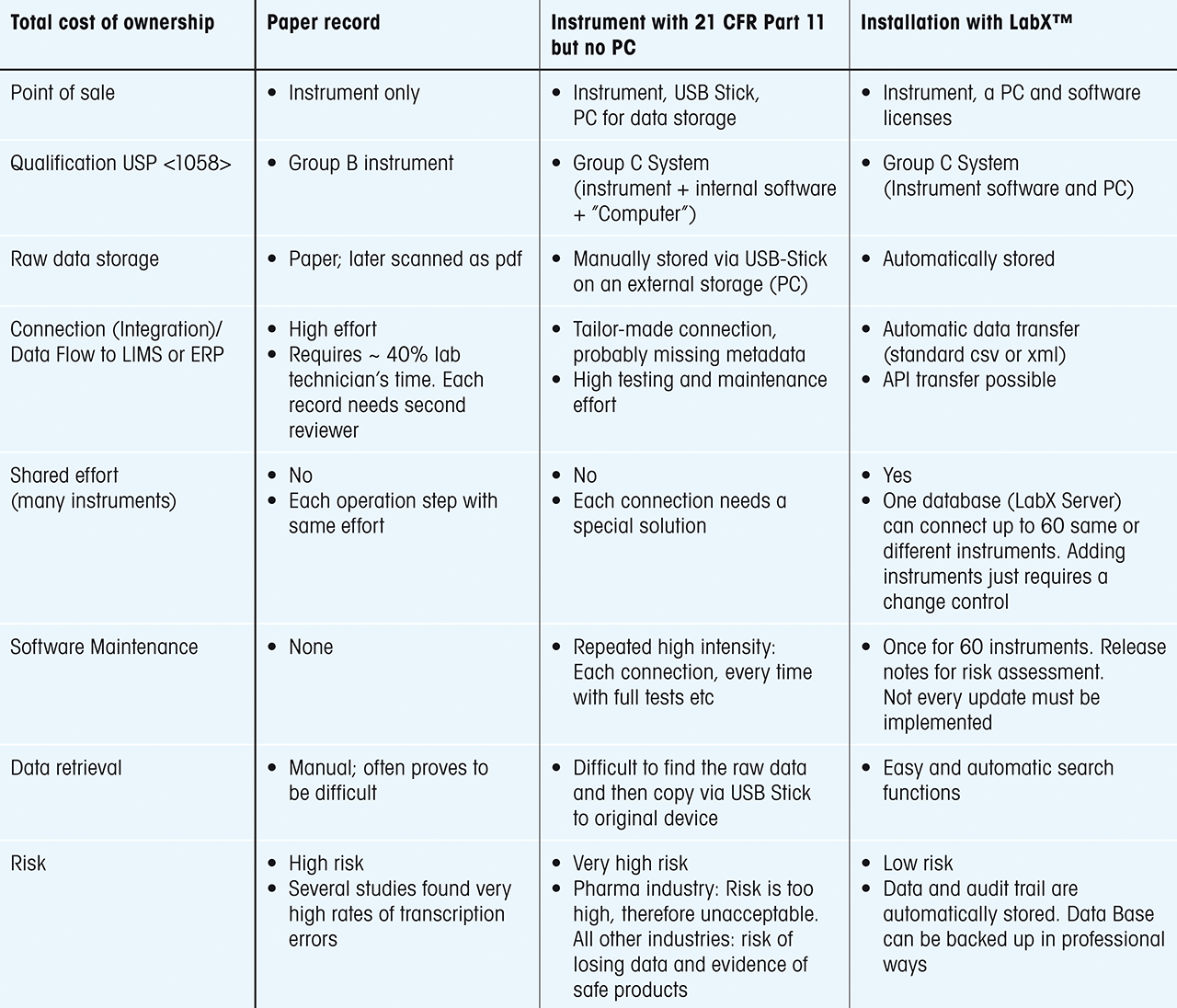 |
Table 1: It is important to assess the total cost of ownership (TCO) not only the costs at “point of sale” (POS). Paper records, or a compromise as discussed above, might be less expensive initially. However, when factoring in routine handling, maintenance, and the risk of data loss, electronic records with LabX® can offer significantly lower costs and less hassle. |
Critical Takeaways and Next Steps
Relying on firmware alone to achieve 21 CFR Part 11 compliance is a risk most labs can’t afford to take. The whitepaper offers an in-depth look at the shortcomings of standalone systems, illustrated by real-world scenarios. As shown in Figure 4 below, it also presents a roadmap for adopting an integrated, software-based model that meets ALCOA++ standards head-on, safeguarding both data integrity and your lab’s reputation.
 |
Figure 4: The LabX software ensures automatic data capture and storage and audit trail readiness for many instruments in a laboratory setting. Instruments are connected through a common network thereby reducing the costs and efforts of validation. The transfer of results, including metadata, to various informatics systems (e.g. LIMS, ERP) are organized and maintained from a shared business server. |
Ready to Dive Deeper?
For a comprehensive exploration of 21 CFR Part 11 compliance without a PC, including expert commentary and practical guidance, download our full whitepaper.
 |
Nuala Nic Ghearailt |
Nuala Nic Ghearailt, Marketing Manager for LabX at METTLER TOLEDO, helps labs boost efficiency, productivity, and compliance through digitalization.
With a background in marketing, sales and strategy management consulting, Nuala brings a strategic overview to our go-to-market approach, while also being able to translate complex concepts into informative communication campaigns.